We're not content to settle when it comes to improving outcomes for the millions of people in the US who are living with HS.
At MoonLake Immunotherapeutics, we are researching Nanobody® approaches.
At MoonLake Immunotherapeutics, we're proud to be the first company to select HiSCR75* as a primary endpoint in our clinical research into hidradenitis suppurativa (HS).1-4
Visit ClinicalTrials.gov to learn more about our ongoing clinical trials in HS.
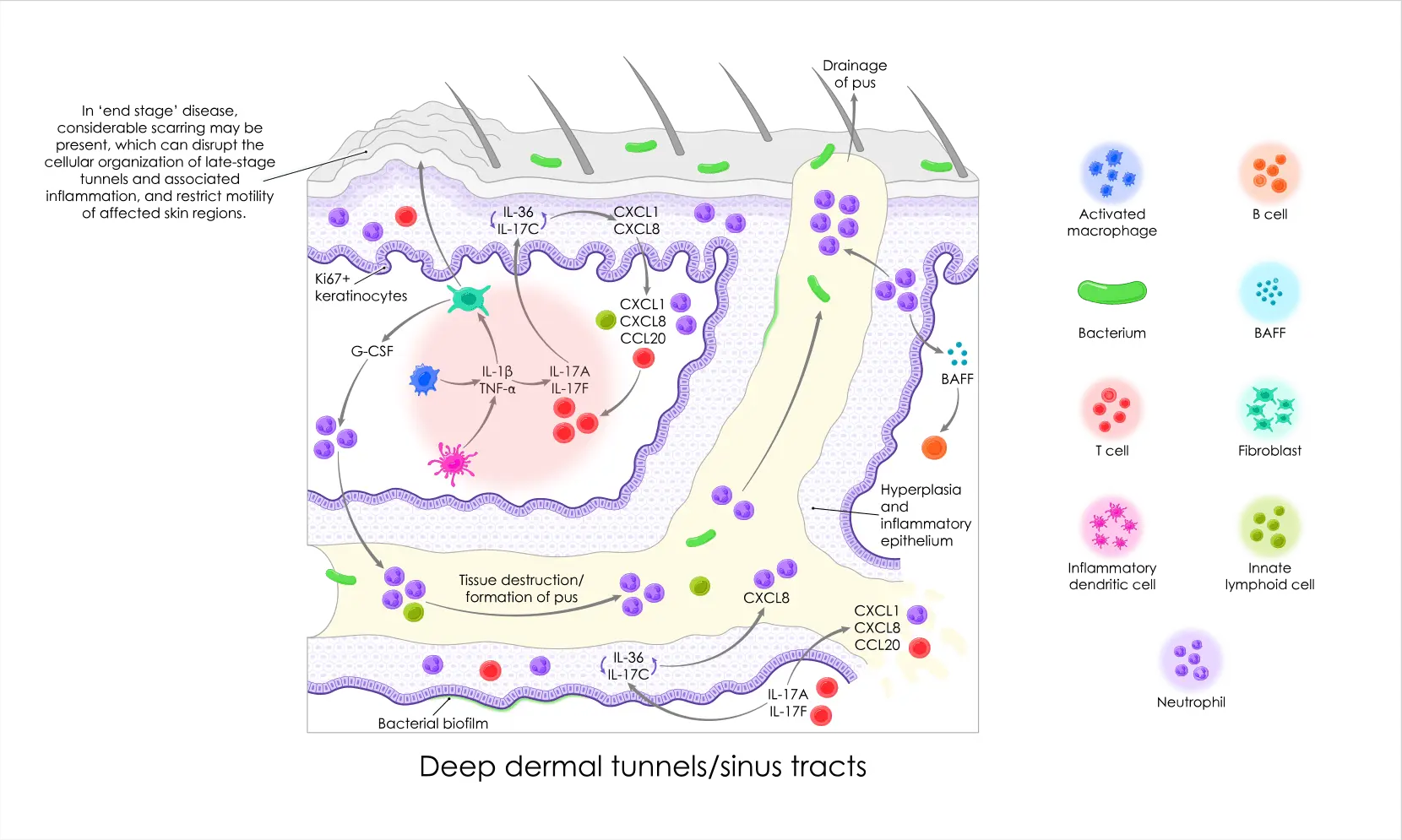
HS is a severely debilitating chronic skin condition resulting in irreversible tissue destruction.5,6
HS manifests as painful inflammatory skin lesions such as nodules and abscesses, typically around the armpits, groin, and buttocks. Over time, uncontrolled and inadequately-treated inflammation can result in the formation of draining dermal tunnels and scarring.5,6
*95% CI, 1.8-3.5%. Based on a systematic review and meta-analysis of studies using a validated questionnaire. †Based on healthcare insurance claims data extrapolated to the full US population.
In a survey of 1,299 patients with HS:*10


Patients report that their HS pain limits their mobility and sleep, harms their psychological wellbeing, and impairs their social relationships.11
*Self-reported HS pain over the past week using a numeric rating scale.







*At any time. By International Classification of Diseases chapters.

Patients with HS also face negative impacts to multiple aspects of life, including impaired work productivity, impaired leisure activity, and sexual dysfunction.12
We’re working to unlock the full potential
of IL-17A and IL-17F inhibition.
Multiple immunological pathways—including the IL-17 pathway—have important roles in inflammatory conditions such as HS.6,13
The proinflammatory cytokines IL-17A and IL-17F are both highly elevated in HS lesions.6
This provides a rationale for the established approach of targeting both IL-17A and IL-17F to interrupt the HS inflammatory cascade.6,13
If left unchecked, this cascade can lead to irreversible tissue destruction, including the formation of deep dermal inflammatory lesions such as abscesses and draining tunnels.6
We’re researching ways to build on the established understanding of IL-17 inhibition and exploring the role of deep tissue penetration in addressing inflammation.6,14
At MoonLake Immunotherapeutics, we are researching Nanobody® approaches.

We're researching the importance of tissue penetration to address deep inflammation in diseases such as HS.14
We're not content to settle when it comes to improving outcomes for the millions of people in the US who are living with HS.
HiSCR75, HS clinical response 75 (≥75% reduction in total abscess and inflammatory nodule count with no increase in abscess or draining tunnel count relative to baseline2–4); HS, hidradenitis suppurativa;
VVH, Heavy chain variable domain from a heavy-chain-only antibody.
Nanobody® and Nanobodies® are registered trademarks of Ablynx, a Sanofi company.
Date of preparation: February 2025